Klinikum rechts der Isar Technische Universität München
Neuro-Kopf-Zentrum
Abteilung für Neuroradiologie
Forschungsgruppen und Projekte
Cellular underpinnings of neurovascular coupling in health and disease
Summary
To meet the energy demand of the brain, it has to be supplied with oxygen and nutrients via the bloodstream. The energy consumption of distinct brain regions is not constant but dependent on their activity status. To accommodate this dynamic demand of metabolic substrates, transient neuronal activity causes localized changes in cerebral blood flow, a process called neurovascular coupling. The ensuing change in blood oxygenation and other hemodynamic parameters can be measured using specialized functional magnetic resonance imaging (fMRI) techniques. Our goal is to better understand the underlying cellular mechanisms linking the neuronal activity to the hemodynamic response in order to allow for a more precise interpretation of fMRI data. We thus use cutting-edge in vivo microscopy techniques and electrophysiology in experimental models to investigate neurovascular coupling and its breakdown in diseases with altered neuronal activity patterns such as Alzheimer’s disease.
Projects
- Investigation of cellular underpinnings of neurovascular coupling in the healthy brain.
- Impaired activity and neurovascular coupling in Alzheimer’s disease
Funding
- Kommission für Klinische Forschung (KKF)
- TUM IAS (Albrecht Struppler Clinician Scientist Fellowship)
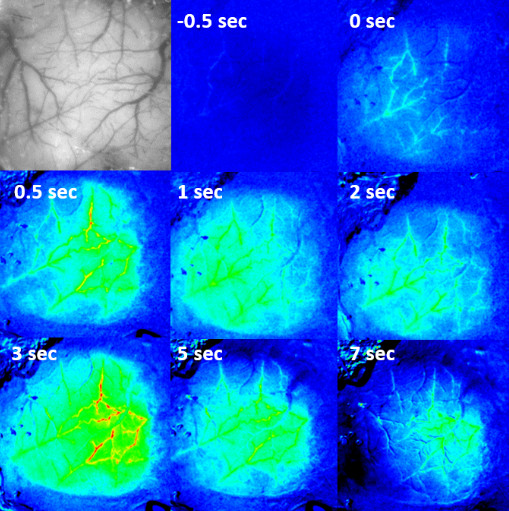 Figure: Hemodynamic response in the primary visual cortex to a visual stimulus (a white LED in front of the contralateral eye flickering at 40 Hz) presented at 0 sec. False-color images normalized to the maximum signal.
Figure: Hemodynamic response in the primary visual cortex to a visual stimulus (a white LED in front of the contralateral eye flickering at 40 Hz) presented at 0 sec. False-color images normalized to the maximum signal.
Lead
Team
Pascal Rossignol;
Juliana Zimmermann (joint with Christian Sorg lab)
Partners
- PD Dr. Christian Sorg, PD Dr. Christine Preibisch (Neuroradiology, TUM)
- Prof. Dr. Arthur Konnerth (Institute for Neuroscience, TUM)
- Prof. Dr. Arne Skerra (Chair of Biological Chemistry, TUM Weihenstephan)
- PD Dr. Matthias Brendel (Nuclear Medicine, LMU)
Selected Publications
BIECHELE, G., BLUME, T., DEUSSING, M., ZOTT, B., SHI, Y., XIANG, X., FRANZMEIER, N., KLEINBERGER, G., PETERS, F., OCHS, K., FOCKE, C., SACHER, C., WIND, K., SCHMIDT, C., LINDNER, S., GILDEHAUS, F.-J., ECKENWEBER, F., BEYER, L., VON UNGERN-STERNBERG, B., BARTENSTEIN, P., BAUMANN, K., DOROSTKAR, M. M., ROMINGER, A., CUMMING, P., WILLEM, M., ADELSBERGER, H., HERMS, J. & BRENDEL, M. 2021. Pre-therapeutic microglia activation and sex determine therapy effects of chronic immunomodulation. Theranostics, 11, 8964-8976.
BIECHELE, G., WIND, K., BLUME, T., SACHER, C., BEYER, L., ECKENWEBER, F., FRANZMEIER, N., EWERS, M., ZOTT, B., LINDNER, S., GILDEHAUS, F.-J., VON UNGERN-STERNBERG, B., TAHIROVIC, S., WILLEM, M., BARTENSTEIN, P., CUMMING, P., ROMINGER, A., HERMS, J. & BRENDEL, M. 2020. Microglial Activation in the Right Amygdala-Entorhinal-Hippocampal Complex is Associated with Preserved Spatial Learning in AppNL-G-F mice. NeuroImage, 117707.
BLUME, T., DEUSSING, M., BIECHELE, G., PETERS, F., ZOTT, B., SCHMIDT, C., FRANZMEIER, N., WIND, K., ECKENWEBER, F., SACHER, C., SHI, Y., OCHS, K., KLEINBERGER, G., XIANG, X., FOCKE, C., LINDNER, S., GILDEHAUS, F.-J., BEYER, L., VON UNGERN-STERNBERG, B., BARTENSTEIN, P., BAUMANN, K., ADELSBERGER, H., ROMINGER, A., CUMMING, P., WILLEM, M., DOROSTKAR, M. M., HERMS, J. & BRENDEL, M. 2021. Chronic PPARγ Stimulation Shifts Amyloidosis to Higher Fibrillarity but Improves Cognition. bioRxiv, 2021.05.30.446348.
FOCKE, C., BLUME, T., ZOTT, B., SHI, Y., DEUSSING, M., PETERS, F., SCHMIDT, C., KLEINBERGER, G., LINDNER, S., GILDEHAUS, F.-J., BEYER, L., VON UNGERN-STERNBERG, B., BARTENSTEIN, P., OZMEN, L., BAUMANN, K., DOROSTKAR, M. M., HAASS, C., ADELSBERGER, H., HERMS, J., ROMINGER, A. & BRENDEL, M. 2018. Early and longitudinal microglial activation but not amyloid accumulation predict cognitive outcome in PS2APP mice. Journal of Nuclear Medicine, 118, 217703
HARTMANN, S., ZHENG, F., CONSTANZE KYNCL, M., KARCH, S., VOELKL, K., ZOTT, B., D'AVANZO, C., LOMOIO, S., TESCO, G., YEON KIM, D., ALZHEIMER, C. & HUTH, T. 2018. β-Secretase BACE1 promotes surface expression and function of Kv3.4 at hippocampal mossy fiber synapses. The Journal of Neuroscience, 38, 3480-3494.
KESKIN, A. D., KEKUS, M., ADELSBERGER, H., NEUMANN, U., SHIMSHEK, D. R., SONG, B., ZOTT, B., PENG, T., FORSTL, H., STAUFENBIEL, M., NELKEN, I., SAKMANN, B., KONNERTH, A. & BUSCHE, M. A. 2017. BACE inhibition-dependent repair of Alzheimer's pathophysiology. Proc Natl Acad Sci U S A, 114, 8631-8636.
QIN, H., FU, L., HU, B., LIAO, X., LU, J., HE, W., LIANG, S., ZHANG, K., LI, R., YAO, J., YAN, J., CHEN, H., JIA, H., ZOTT, B., KONNERTH, A. & CHEN, X. 2018. A Visual-Cue-Dependent Memory Circuit for Place Navigation. Neuron, 99, 47-55.e4.
ZOTT, B., BUSCHE, M. A., SPERLING, R. A. & KONNERTH, A. 2018. What Happens with the Circuit in Alzheimer’s Disease in Mice and Humans? Annual Review of Neuroscience, 41, 277-97.
ZOTT, B., SIMON, M. M., HONG, W., UNGER, F., CHEN-ENGERER, H.-J., FROSCH, M. P., SAKMANN, B., WALSH, D. M. & KONNERTH, A. 2019. A vicious cycle of β amyloid–dependent neuronal hyperactivation. Science, 365, 559-565.